| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-17 19:28:51 UTC |
|---|
| Updated at | 2020-12-07 19:11:54 UTC |
|---|
| CannabisDB ID | CDB005295 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | 3,7-Dimethyluric acid |
|---|
| Description | 3,7-Dimethyluric acid, also known as 3,7-dimethylate or 3,7-DMU, belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. An oxopurine that is 7,9-dihydro-1H-purine-2,6,8(3H)-trione substituted by methyl groups at N-3 and N-7. 3,7-Dimethyluric acid is an extremely weak basic (essentially neutral) compound (based on its pKa). 3,7-Dimethyluric acid exists in all living organisms, ranging from bacteria to humans. 3,7-dimethyluric acid can be biosynthesized from theobromine through the action of the enzyme xanthine dehydrogenase/oxidase. In humans, 3,7-dimethyluric acid is involved in caffeine metabolism. 3,7-Dimethyluric acid is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,7-Dimethylate | Generator | | 3,7-Dimethylic acid | Generator | | 37-Dimethyluric acid | ChEMBL, HMDB | | 37-Dimethylate | Generator, HMDB | | 37-Dimethylic acid | Generator, HMDB | | 3,7-Dimethyl-2,6,8-trihydroxypurine | HMDB | | 3,7-Dimethyl-7,9-dihydro-1H-purine-2,6,8(3H)-trione | HMDB | | 3,7-DMU | MeSH, HMDB |
|
|---|
| Chemical Formula | C7H8N4O3 |
|---|
| Average Molecular Weight | 196.16 |
|---|
| Monoisotopic Molecular Weight | 196.0596 |
|---|
| IUPAC Name | 3,7-dimethyl-2,3,6,7,8,9-hexahydro-1H-purine-2,6,8-trione |
|---|
| Traditional Name | 3,7-dimethyluric acid |
|---|
| CAS Registry Number | 13087-49-5 |
|---|
| SMILES | CN1C(=O)NC2=C1C(=O)NC(=O)N2C |
|---|
| InChI Identifier | InChI=1S/C7H8N4O3/c1-10-3-4(8-6(10)13)11(2)7(14)9-5(3)12/h1-2H3,(H,8,13)(H,9,12,14) |
|---|
| InChI Key | HMLZLHKHNBLLJD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Xanthines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthine
- 6-oxopurine
- Purinone
- Alkaloid or derivatives
- Pyrimidone
- N-substituted imidazole
- Pyrimidine
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Urea
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Disposition | Source: Biological location: |
|---|
| Role | Biological role: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | logP | -1.33 | GASPARI,F & BONATI,M (1987) |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | 3,7-Dimethyluric acid, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0v0a-0900000000-46adc1bd1a2829ed3eab | Spectrum | | Predicted GC-MS | 3,7-Dimethyluric acid, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0002-0900000000-5554059238ba6f5a4aa7 | 2012-07-25 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-001l-9800000000-bcef3be559d5d3540d62 | 2012-07-25 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-014l-9000000000-7d225864dafe7484ef17 | 2012-07-25 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-81b25f479c4f1663781d | 2017-09-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0007-0900000000-0f875011e8751037af3f | 2017-09-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-ddcf3e38bdc4d7198abc | 2017-09-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-f9c0039adcc98c79f10d | 2017-09-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6t-0900000000-5398ccddcd1ad6de4811 | 2017-09-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-79381deb2ab0e04daf89 | 2017-09-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-0e499ff6f23e328dc54a | 2021-09-21 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-c0e1ff53101ddbba850d | 2021-09-21 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9300000000-a0758f9ce9eb83d7a97d | 2021-09-21 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-8ba3918ee48755b0ba1a | 2021-09-25 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-2900000000-a5948ea7307823697f8e | 2021-09-25 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-82b93be35cae293c6cab | 2021-09-25 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Caffeine Metabolism |    | 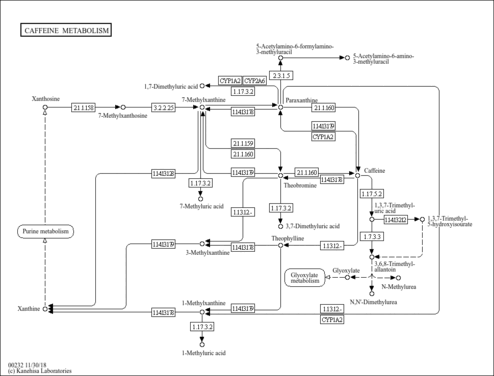 |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0001982 |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB022780 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 74994 |
|---|
| KEGG Compound ID | C16360 |
|---|
| BioCyc ID | CPD-12482 |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 83126 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 68531 |
|---|
| References |
|---|
| General References | Not Available |
|---|
